The road to registration
Your success is our mission. Benefit from our out-standing expertise to face up to the regulatory obligations for plant protection products, biocides, biostimulants and adjuvants in Europe.
HIGHEST QUALITY SINCE 2003
Who we are
Linge Agroconsultancy is an independent consulting company with a strong team of experienced specialists to provide an extensive service portfolio to secure registration of your active substances and products in all European countries. Get to know us!
- Linge Agroconsultancy B.V. Oosterhoutsestraat 95 6678 PG Oosterhout (Gld) The Netherlands

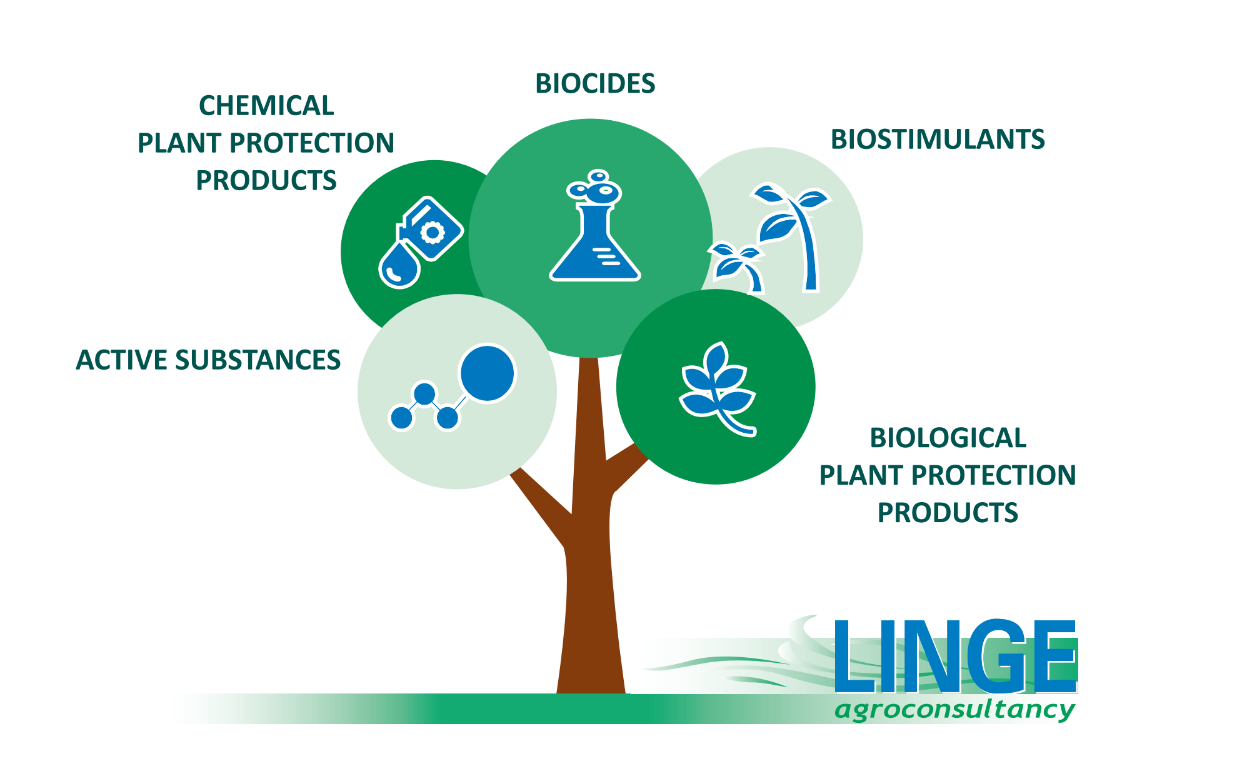
All actives and product types
Our services
All EU countries and tasks
All EU nations
- Active substance registration
- IUCLID dossier
- Zonal applications
- Interzonal applications
- National applications
- DGAs
- and many more
A team of experienced specialists
Our History
Linge agroconsultancy is a small and flexible consulting company founded in 2003. Together with our strong partners for field, laboratory and greenhouse trials and studies we are able to serve all your needs and ensure a smoother registration process and a faster entry of your product onto the market.
Our team of experienced specialists provides a full service to secure registration of your active substances and products. Confidentiality is, of course, guaranteed.
We carry out assignments for both, government and industry and act as an evaluator for the competent authority in the Netherlands. Our short communication lines and customer-oriented work make us an ideal partner for your registrations in Europe.
Our key competencies are registration of plant protection products in the framework of Regulation (EC) No 1107/2009, registration of biocides following the framework laid out in Regulation (EU) No 528/2012, as well as registration of adjuvants and biostimulants and management of task forces. Discover our comprehensive service portfolio.
We carry out assignments for both, government and industry and act as an evaluator for the competent authority in the Netherlands. Our short communication lines and customer-oriented work make us an ideal partner for your registrations in Europe.
Our key competencies are registration of plant protection products in the framework of Regulation (EC) No 1107/2009, registration of biocides following the framework laid out in Regulation (EU) No 528/2012, as well as registration of adjuvants and biostimulants and management of task forces. Discover our comprehensive service portfolio.



